Characteristic symptoms of plant deficiencies part 2
Depending on the location of symptoms on the plant, deficiencies can be divided into two categories:
1) When the symptoms are first observed in the leaves at the base of the plant and with time progress upwards, even reaching the leaves at the top, then we speak of nitrogen, phosphorus, potassium and magnesium deficiencies. When we see this, the young leaves of the plant take up the necessary elements from the lower leaves, resulting first in the appearance of symptoms and then in the drying out of these base leaves. If the cause is not treated, these symptoms progress to the leaves above.
2) In the second category are deficiencies whose symptoms are first seen in the top leaves and then progress downward. This category includes deficiencies caused by deficiencies of calcium, boron, manganese, copper and iron.
Calcium deficiency, boron deficiency
3. When the symptoms are localized or more pronounced in young leaves and parts of the shoots and there is necrosis of the apical buds, this is called calcium and boron deficiency.
Calcium deficiency
There is deformation of the young leaves, chlorosis and necrosis occurs either at the top or at the periphery. The tips of the roots show a slight swelling and the fruits show necrotic spots.
Boron deficiency
The color of the leaves changes from light green to yellow. Smaller than normal leaves and deformation are observed. The root system of the plants has limited growth and a brownish tinge.
Figure 9. Calcium deficiency in tomato leaves.
Figure 10. Calcium deficiency in tomato fruits.
Figure 11. Healthy tomato plant on the left and right with boron deficiency.
Iron deficiency, manganese deficiency, zinc deficiency
4. When the top bud of the shoots does not die, in this case we are dealing with iron, manganese and zinc deficiencies.
Iron deficiency
Figure 12. Iron deficiency.
In the beginning we have a network of green ribs but in advanced deficiency the color of the leaf becomes yellow to yellowish-white. Rarely, however, necrosis of the leaf will occur.
Manganese deficiency
There is chlorosis between the nerves, but this is not as pronounced as in iron deficiency. At an advanced stage, the chlorotic tissues take on a brownish tinge and scattered spots appear.
Zinc deficiency
Smaller than normal leaves and chlorotic spotting or uniform reduction in the green color of the leaf are the characteristics of this trophic condition and, less frequently, necrosis of the lamina.
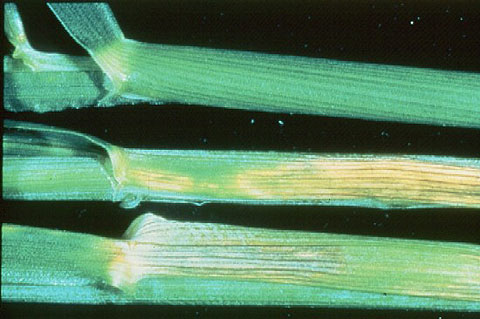
Figure 13. Zinc deficiency in corn.
Figure 14. Zinc deficiency in wheat.
Nutrition from the leaves of the plant
Plant nutrition from the leaves depends on:
- on the nutrient and
- on the leaf
The epidermis and the age of the leaf determine:
- a) the rate of nutrition uptake and
- b) the rate of ion movement.
The structure and chemical composition of the epidermis, the thickness and chemical composition of the epithelium.
The tegument is the continuous membrane, which is located on the epidermal cells of leaves and covers various cavities.
It is a layer composed of cell wall materials, over which there are two layers, an inner and an outer layer.
Above the tegument is a waxy layer and the moisture on the epidermis are key factors in determining the rate at which elements are taken up.
The main role of the existence of the tegument is considered to be the limitation of water loss by transpiration.
An important role is played by the membrane in protecting the plant from pathogenic micro-organisms. The tegument appears as a barrier that prevents the entry of viruses, bacteria, fungi and sometimes insects.
The environment provided by the leaf surface for the growth of organisms was called the phyllosphere. Bacteria such as nitrogen-fixing bacteria and fungi grow on it.
Subsequently, colonies of other organisms can also grow, with the result that there is competition between them for the nutrients secreted by the leaf.
Agrochemicals are all synthetic substances used in agriculture.
They include chemical fertilisers, pesticides, insecticides, fungicides and hormones. In general, they are any substance that helps to improve plant production, such as those used to nourish plants, which we will discuss a little later. More specifically, nutrient agrochemicals or fertilizers are the class of agrochemicals that provide the plant with the elements necessary for its nutrition.
We can divide agrochemicals for nutrition as follows: conventional, which are solid and liquid, slow release fertilizers, chelated fertilizers and other fertilizers, which are still in the research stage.
The way we give our plants the agrochemicals to feed them is what we know as fertilization, we’ve talked a lot lately about fertilization, I don’t think you have any complaints. And it’s done in two ways: either from the root, so we’re talking about soil fertilization, or from the leaves, so we have transfoliar fertilization.
The conventional fertilizers
They are the inorganic and organic fertilizers. For more information, see the article:“Industrial fertilizers and fertilization with manure, compost, peat, green manure, crop rotation, soil“.
Slow release fertilizers
Many times quantities of fertilizers are not available to plants. While they initially absorb a large amount immediately, the supply is gradually reduced. The need then arises for slow or controlled release fertilizers, where they have a continuous and steady flow. Converting conventional fertilizers to slow-release fertilizers is achieved in several ways and aims at the following: to be able to control the supply of nutrients, not to give our plants more than they need, to avoid “leaching” of nutrients from the soil and to improve the soil.
How can you convert a conventional fertilizer into a slow release fertilizer? If you coat it with a material or mixture of materials and thus the nutrients are clearly released at a slower rate. In this way, what you are making consists of both fertilizer elements and non-fertilizer elements. We know how fertilizing elements contribute, so it remains for us to see how non-fertilizing elements work: they reduce losses, improve the physical properties of the fertilizing elements and contribute to their gradual yield in plants. A key requirement for non-fertilising elements to work is that they must be non-toxic to the environment. Mastic, bitumen, fatty acids, etc. have been used. For example, urea-aldehyde fertilizer has been used, where it provides nitrogen to crops for a long time.
The chelated compounds
Chemical compounds are complex compounds consisting of an organic molecule and a metal, trace element and others. The name chelate is derived from the word chelate meaning claw.
For a chelating agent to be used as a soil-administered agrochemical nutrient, it must exhibit the following properties: the chelating agent must be water-soluble, the metal it contains must not be replaced by another in the soil, it must not be affected by soil microorganisms, and it must not be toxic to plants.
Various chelating agents have been used in agriculture, such as ethylene-diamine-tetraacetic acid, diethylene-triamine-pentaacetic acid, cyclohexanediamine-tetraacetic acid, etc.
The treatment of food deficiencies with agrochemical nutrients
The treatment of food deficiencies is achieved when plants are adequately supplied with the necessary nutrients. To this end, the nutrients are administered to the sick plants in a suitable manner. Nutrient misuse can create imbalances in the plant, leading to the development of other food deficiencies or even toxicity. The threshold beyond which the negative effect starts varies from element to element as well as from crop type. The amounts of nutrients applied should be increased when soil conditions allow their fixation. In cases where nutrients are soon inactivated in the soil so that the necessary amount cannot be absorbed by the root, it is necessary to apply them by spraying.
The treatment of plants is therefore not achieved in the same way in all cases of food deficiencies. The basic condition for treating a food deficiency is that there must not be a single limiting factor affecting the absorption of nutrients, such as water deficiency or poor ventilation. The sources of nutrients are usually various soluble minerals which are incorporated into the soil or dissolved in water and sprayed onto the weak plants. In trees, nutrients may be injected into the trunk by dry or liquid injection. This method is effective for the treatment of trace element deficiencies, but it is not practical and only recommended in special cases.
Fertilisation with nitrogen, phosphorus and potassium
The elements are given in the form of the usual fertilisers which are spread and incorporated into the soil. These elements are not usually applied by spraying because even in cases where there is a positive reaction of the plants, a large number of treatments are required to meet their needs. An exception is nitrogen when applied in the form of urea. Urea is very easily absorbed by the leaves and is a satisfactory source of nitrogen for the plant.
Calcium fertilisation
In acidic soils, calcareous materials such as limestone are added, which at the same time regulate the pH. In soils with a neutral or alkaline reaction, calcium enrichment is achieved by adding gypsum (calcium sulphate). To prevent fruit diseases caused by insufficient calcium supply, the fruit is repeatedly soaked with an aqueous solution of calcium chloride or calcium nitrate.
Fertilisation with magnesium
Magnesium is incorporated into the soil or applied by spraying. In neutral and alkaline soils, magnesium sulphate is used, while in acidic soils, dolomite limestone is preferred, with which acidity is corrected at the same time. Foliar application is not equally effective for all plant species. For this purpose, magnesium sulphate solutions of 1-3 % are used, with which the plants are sprayed two to three times at the beginning of the growing season. More effective are sprays with magnesium nitrate solutions of 0.5-0.75%.
Sulphur fertilisation
Under normal growing conditions there is no sulphur deficiency and therefore no need for separate application of sulphur. The various fertilisers used in crops contain a considerable amount of sulphate, while significant amounts of sulphur enter the soil from sulphur-containing pesticides or are absorbed by the leaves as sulphur dioxide (SO2) from the atmosphere.
Iron fertilisation
The main cause of nutrient deficiency is the inability of the soil to retain sufficient quantities of soluble iron to meet the needs of growing plants. The addition of inorganic iron salts to the soil is not effective, because iron is soon converted into insoluble compounds. But spraying the foliage with iron sulphate does not give satisfactory results either. There are, however, chelated iron compounds, the use of which avoids its precipitation in insoluble form. The root has the ability to absorb the iron chelates, which are then broken down and release assimilable iron into the plant. The iron content of commercially available synthetic chelates ranges from 6-12 % and is applied by spraying or soil addition.
For spraying, aqueous solutions of 0.1% chelating agent are used. Young leaves turn green within a few days after spraying. In general, however, chelating agents are not very effective when administered from the leaves. On the contrary, their incorporation into the soil always gives good results and diseased plants turn normal green within a few weeks. The amount of chelating agent given from the soil varies according to the soil composition, the size of the plant and its chemical structure. In mature trees 100-400 g/tree, in shrubs 10-20 g/plant and in small herbaceous plants 5-10 g/m2 of soil surface are applied. The effect of iron chelates does not last longer than one growing season and the application should be repeated every year.
In addition to iron, other metals that are plant nutrients also form chelating compounds. These compounds do not give better results than the inorganic forms of the elements for the treatment of food deficiencies and their use in practice is very limited.
Manganese fertilisation
Manganese deficiency is usually due to the prevalence in the soil of conditions favourable to the fixation of this element and therefore the addition of manganese sulphate or other inorganic salts of manganese does not give good results. Thus, the supply of manganese to plants is achieved by spraying the foliage with a 0,1-0,5 % solution of manganese sulphate, which is carried out 1-2 times during the spring period.
Zinc fertilization
The addition of zinc salts to the soil also does not give good results, and the supply of zinc to plants is usually carried out by spraying the aboveground part. Vegetables and other annual plants are wetted 1-2 times during their growing period with a zinc sulphate solution of 0.05-0.5%. Deciduous trees are sprayed during the dormant period with a zinc sulphate solution of 2-4%. Spraying during the germination period is carried out with a more dilute solution, but it is not as effective and often causes burns on the fruit. Citrus trees are sprayed at the end of spring with a 0,5 % zinc sulphate solution, which must necessarily be neutralised with lime to avoid burns.
Copper fertilisation
Copper deficiency is rare and is easily treated by spraying the foliage with a dilute solution of copper sulphate, which must be neutralised with lime. Deciduous trees can be sprayed in winter during the dormant period with a relatively concentrated solution of sulphate (2-3 %).
Boron fertilisation
The difference between normal and toxic amounts of boron is relatively small and special care must be taken when this nutrient is supplied to plants. Borax Na2B4O710H2Oand boric acid H3BO3, as well as other boron-containing materials, are used as sources of boron. Borax is most commonly used for adding boron to the soil, while boric acid is used for foliar application by spraying. The amount of boron incorporated into the soil depends on the type of crop and the composition of the soil. Some plant species are particularly susceptible to excess boron and therefore in cases of food deficiency should receive the lowest effective dose of boron, especially in sandy soils. As an indication, the amounts of borax added to the soil are 1-4 kg/ha for annual crops and 100-300 g/tree for fruit trees. The addition of boron to the soil usually takes longer than one growing season and should therefore not be repeated every year because of the risk of boron accumulation in plants. Spraying the foliage with a 0,1-0,2 % solution of boric acid supplies the plants with boron, but its effect lasts only one growing season.
Molybdenum fertilisation
The sources of molybdenum are sodium molybdate Na2MoO42H2Oand ammonium molybdate (NH4)6Mo7O244H2O, which are incorporated into the soil or sprayed. The molybdenum requirements of plants are very low and therefore minimal amounts of these salts are sufficient to cure food deficiency. The amounts of molybdate added to the soil are in the range of 25-80 g/ha, and foliar sprays are applied at 60-120 ppm. Food deficiency usually occurs in acidic soils and often liming to adjust acidity results in an increase in available molybdenum in sufficient quantities to meet plant needs. Since the presence of high concentrations of molybdenum in plants used as feed causes disturbances in animals, particular care is required when this element is applied to fodder crops.
Fertilisation from the leaves
See article:“Soil, fertilizers and plant nutrients next“… In addition, the following have been applied transgallantly: urea, iron sulphate, iron chelate, magnesium sulphate, manganese sulphate, copper sulphate, zinc sulphate, zinc sulphide, zinc carbonate and sodium molybdate.
Transfertilisation is most effective when the uptake of elements by the roots is limited and is favoured by the presence of rich foliage, such as in fruit trees. Cereals, due to their small foliage, especially in early spring, do not benefit from transhumance. The response of crops to transhumance is faster, but also more temporary than that of soil fertilisation.
Of the nitrogen fertilizers, only urea showed good results as a transfertilizer. The other nitrogen fertilizers such as (sodium nitrate) NaNO3 and ammonium sulphate (NH4).2SO4 cause leaf burn. Urea increases the permeability of the membrane and thus facilitates the entry of ever larger quantities of urea. Iron sulphate enters more rapidly in the presence of urea than in its absence, but the movement of iron into the leaf is greater when it is chelated. Of the phosphates, orthophosphate is the most effective trans-fertilizer.
Potassium transfoliate fertilizers cause burns and are therefore not applied. Besides, the potassium requirements of the growing plant are too high to be met by transhumance. Fortunately, transhumance with magnesium is used. Magnesium fertilizations through the soil can take up to three years to correct magnesium deficiency symptoms in trees, while spraying with a 2% MgSO4 solution corrects the symptoms within a few days.
Iron uptake from the leaves is also possible. However, it appears that iron is not easily moved into the foliage. In addition, the presence of high levels of phosphate in the foliage helps its easy precipitation, especially if the spray solution is approximately neutral. Iron sulphate is recommended as an intergrowth fertilizer. The addition of manganese to the soil is not effective and there is a general problem of oxidation of the added manganese in the soil. On the contrary, transhumance with manganese fertilization gives fast and good results and is recommended for alkaline calcareous soils. Of the manganese salts, manganese sulphate is successfully used as a transfertilizer. Copper (as copper sulphate), zinc (as sulphate, sulphate and carbonate) and finally molybdenum (as sodium molybdate) are successfully used as interplant fertilizers.
An article by Sophia Papazoglou
Agricultural Engineer – MSc Environmental Management
Part 1
The first part of the article can be found at “The diagnosis of the nutritional status of the plant – Part 1/2“.
Tags: DEFICIENCIES • DEFICIENCY • DIAGNOSIS • FERTLIZERS • FOLIAR DIAGNOSIS • NUTRIENTS • SOFIA PAPAZOGLOU AGRONOMIST • SYMPTOMS